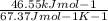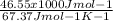Chemistry, 15.03.2020 07:03 christingle2004
The ΔH vap of a certain compound is 46.55 kJ⋅ mol−1 and its ΔS vap is 67.37 J⋅mol−1⋅K−1.
What is the boiling point of this compound?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2
You know the right answer?
The ΔH vap of a certain compound is 46.55 kJ⋅ mol−1 and its ΔS vap is 67.37 J⋅mol−1⋅K−1.
What...
What...
Questions


Social Studies, 04.10.2020 14:01



Mathematics, 04.10.2020 14:01


History, 04.10.2020 14:01

Mathematics, 04.10.2020 14:01


Mathematics, 04.10.2020 14:01

English, 04.10.2020 14:01


Biology, 04.10.2020 14:01

History, 04.10.2020 14:01






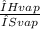
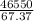
 C
C