
Chemistry, 09.03.2020 17:02 Kalle91106
Quantum numbers arise naturally from the mathematics used to describe the possible states of an electron in an atom.
The four quantum numbers, the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms) have strict rules which govern the possible values.
Identity allowable combinations of quantum numbers for an electron. Select all that apply.
a) n = 3, l = 2, ml = −2, ms = −1/2
b) n = 4, l = 0, ml = 1, ms = −1/2
c) n = 3, l = −2, ml = −2, ms = +1/2
d) n = 5, l = 4, ml = 4, ms = +1/2
e) n = 3, l = 3, ml = 1, ms = +1/2
f) n = 2, l = 1, ml = 0 ,ms = 0

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1


Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
Quantum numbers arise naturally from the mathematics used to describe the possible states of an elec...
Questions


Social Studies, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

Spanish, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

Mathematics, 18.03.2021 01:20

English, 18.03.2021 01:20




Social Studies, 18.03.2021 01:20


Biology, 18.03.2021 01:20

Chemistry, 18.03.2021 01:20





Health, 18.03.2021 01:20

English, 18.03.2021 01:20

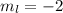 ,
, 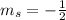
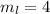 ,
, 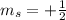
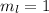 ,
, 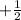 for upward spin and
for upward spin and 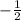 for downward spin.
for downward spin.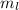 can have values from -l to +l i.e for l= 2 ,
can have values from -l to +l i.e for l= 2 , 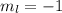 ,
, 

