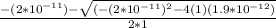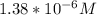Chemistry, 03.03.2020 01:01 ELGuapo6746
Calculate the pH at the equivalence point for the titration of 0.190 M 0.190 M methylamine ( CH 3 NH 2 ) (CH3NH2) with 0.190 M HCl . 0.190 M HCl. The K b Kb of methylamine is 5.0 × 10 − 4 .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1


Chemistry, 23.06.2019 05:00
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 1
You know the right answer?
Calculate the pH at the equivalence point for the titration of 0.190 M 0.190 M methylamine ( CH 3 NH...
Questions


English, 27.11.2019 23:31

Mathematics, 27.11.2019 23:31


History, 27.11.2019 23:31




Mathematics, 27.11.2019 23:31

Mathematics, 27.11.2019 23:31

Social Studies, 27.11.2019 23:31


Mathematics, 27.11.2019 23:31

Biology, 27.11.2019 23:31


Biology, 27.11.2019 23:31


Advanced Placement (AP), 27.11.2019 23:31


Social Studies, 27.11.2019 23:31

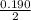
![K_a =\frac{[CH_3NH_2][H^+]}{[CH_3NH^+_3]}](/tpl/images/0531/3181/3871d.png)
![K_a = \frac{[x][x]}{[0.095-x]}](/tpl/images/0531/3181/0ca50.png)
![K_a = \frac{[x^2]}{[0.095-x]}](/tpl/images/0531/3181/85100.png) ------ equation (1)
------ equation (1)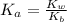
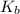 =
= 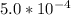 ; and
; and 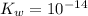 ;
;
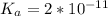
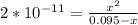
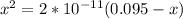
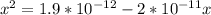

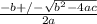 ; we have
; we have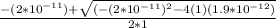 OR
OR