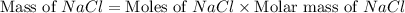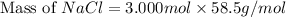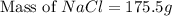Chemistry, 02.03.2020 18:22 squawk1738
The molality equation also requires the moles of solute. The molarity describes 3.000 moles of NaCl per 1 L of solution. Use the moles of NaCl to solve for the mass of NaCl. Note: use the molar mass of NaCl rounded to 4 significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 23.06.2019 00:30
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1

Chemistry, 23.06.2019 11:00
Which example is a mechanical wave? a.microwave b.radio wave c.water wave d.ultraviolet light
Answers: 1
You know the right answer?
The molality equation also requires the moles of solute. The molarity describes 3.000 moles of NaCl...
Questions



Mathematics, 05.05.2020 22:11

Mathematics, 05.05.2020 22:11


Mathematics, 05.05.2020 22:11




Mathematics, 05.05.2020 22:11


Mathematics, 05.05.2020 22:11


English, 05.05.2020 22:11



English, 05.05.2020 22:11

Biology, 05.05.2020 22:11


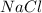 = 3.000 mol
= 3.000 mol