

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

Chemistry, 23.06.2019 14:00
Which word refers to the smallest functional unit of living thing
Answers: 1
You know the right answer?
For the reaction shown, calculate how many moles of NH3 form when 16.72 moles of reactant completely...
Questions


Advanced Placement (AP), 01.07.2019 12:00

Mathematics, 01.07.2019 12:00












Mathematics, 01.07.2019 12:00

Mathematics, 01.07.2019 12:00




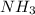 formed are, 22.3 moles.
formed are, 22.3 moles.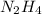 = 16.72 mol
= 16.72 mol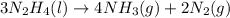
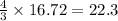 moles of
moles of 

