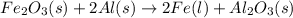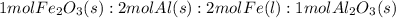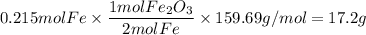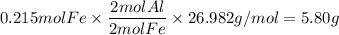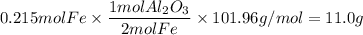Over the years, the thermite reaction has been used for welding railroad rails, in incendiary bombs, and to ignite solid-fuel rocket motors. The reaction is given below. Fe2O3(s) + 2 Al(s) 2 Fe(l) + Al2O3(s) What masses of iron(III) oxide and aluminum must be used to produce 12.0 g iron? iron (III) oxide WebAssign will check your answer for the correct number of significant figures. 17.2 Correct: Your answer is correct. g aluminum WebAssign will check your answer for the correct number of significant figures. 2.98 Incorrect: Your answer is incorrect. g What is the maximum mass of aluminum oxide that could be produced?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1



Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1
You know the right answer?
Over the years, the thermite reaction has been used for welding railroad rails, in incendiary bombs,...
Questions

History, 04.12.2020 23:00

History, 04.12.2020 23:00


Mathematics, 04.12.2020 23:00

Mathematics, 04.12.2020 23:00




Social Studies, 04.12.2020 23:00


Computers and Technology, 04.12.2020 23:00

English, 04.12.2020 23:00

Mathematics, 04.12.2020 23:00

Mathematics, 04.12.2020 23:00

Mathematics, 04.12.2020 23:00

Mathematics, 04.12.2020 23:00

Mathematics, 04.12.2020 23:00

Mathematics, 04.12.2020 23:00


Social Studies, 04.12.2020 23:00