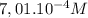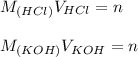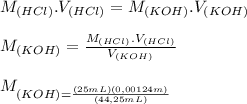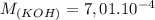Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3


Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1

Chemistry, 23.06.2019 15:30
Sodium chloride can be made as follows: 2na + cl2 ? 2nacl i calculate the maximum amount of nacl possible if 2.3 g of sodium was reacted with excess chlorine. show all your workings.
Answers: 3
You know the right answer?
A student has a 0.00124 M HCl (aq) solution and she titrates 25.00 mL of this solution against an un...
Questions

History, 02.05.2021 16:30

Mathematics, 02.05.2021 16:30

Chemistry, 02.05.2021 16:30


Biology, 02.05.2021 16:40


Mathematics, 02.05.2021 16:40



Mathematics, 02.05.2021 16:40



SAT, 02.05.2021 16:40

Health, 02.05.2021 16:40

Mathematics, 02.05.2021 16:40




Social Studies, 02.05.2021 16:40