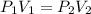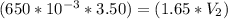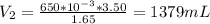Chemistry, 02.03.2020 04:06 letsgetcookingblog
A gas occupies a volume of 650.0 mL when the pressure is 3.50 atm. What will the new volume be if the pressure is reduced to 1.65 atm and the temperature remains constant.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 00:30
This element exists in adundance in the sun.explain how you would go about capturing sunlight.would this captured sunlight contain any of the element?
Answers: 1


Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
A gas occupies a volume of 650.0 mL when the pressure is 3.50 atm. What will the new volume be if th...
Questions

Computers and Technology, 15.07.2020 05:01















Computers and Technology, 15.07.2020 05:01



Mathematics, 15.07.2020 05:01

Mathematics, 15.07.2020 05:01