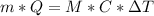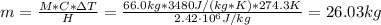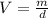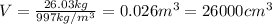The evaporation of sweat is an important mechanism for temperature control in some warm-blooded animals.
a. What mass of water must evaporate from the skin of a 66.0 kg man to cool his body 1.30 °C? The heat of vaporization of water at body temperature (37.0 ∘C) is 2.42×10^6J/kg. The specific heat capacity of a typical human body is 3480 J/(kg⋅K).
b. What volume of water must the man drink to replenish the evaporated water? Compare this result with the volume of a soft-drink can, which is 355 cm^3

Answers: 2


Another question on Physics

Physics, 22.06.2019 05:00
The image below shows numerous volcanic mountains in the pacific northwest. what is the most likely cause of the volcanic and earthquake activity in this region?
Answers: 2

Physics, 22.06.2019 11:00
Consider a system to be two train cars traveling toward each other. what is the total momentum of the system before the train cars collide? kg • what must the total momentum of the system be after the train cars collide? kg •
Answers: 2

Physics, 22.06.2019 11:40
Consider the following position function. find (a) the velocity and the speed of the object and (b) the acceleration of the object. bold r left parenthesis t right parenthesisr(t)equals=left angle 6 t superscript 4 baseline comma 2 t cubed right angle6t4,2t3 for tgreater than or equals≥0
Answers: 3

Physics, 22.06.2019 15:30
An alien spaceship is 500 m above the ground and moving at a constant velocity of 150 m/s upwards. how high above the ground is the ship after 5 seconds?
Answers: 1
You know the right answer?
The evaporation of sweat is an important mechanism for temperature control in some warm-blooded anim...
Questions

Mathematics, 02.02.2020 23:59

English, 02.02.2020 23:59


Biology, 02.02.2020 23:59


Mathematics, 02.02.2020 23:59


Mathematics, 02.02.2020 23:59

Biology, 02.02.2020 23:59



History, 02.02.2020 23:59

Social Studies, 02.02.2020 23:59

Biology, 02.02.2020 23:59


Mathematics, 02.02.2020 23:59

Biology, 02.02.2020 23:59

History, 02.02.2020 23:59