
Chemistry, 28.02.2020 20:53 tammydbrooks43
Standard heats of formation for reactants and products in the reaction below are provided. 2 HA(aq) + MX2(aq) → MA2(aq) + 2 HX(l) Substance ΔHf° (kJ/mol) HA(aq) -357.05 HX(l) -449.579 MA2(aq) -63.958 MX2(aq) 69.602 What is the standard enthalpy of reaction, in kJ? Report your answer to three digits after the decimal.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1


Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Standard heats of formation for reactants and products in the reaction below are provided. 2 HA(aq)...
Questions







Computers and Technology, 26.11.2019 02:31













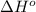
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0528/4508/45485.png)
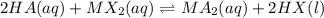
![\Delta H^o_{rxn}=[(n_{(MA_2)}\times \Delta H^o_f_{(MA_2)})+(n_{(HX)}\times \Delta H^o_f_{(HX)})]-[(n_{(HA)}\times \Delta H^o_f_{(HA)})+(n_{(MX_2)}\times \Delta H^o_f_{(MX_2)})]](/tpl/images/0528/4508/6d322.png)
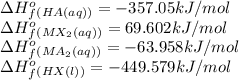
![\Delta H^o_{rxn}=[(1\times -63.958)+(2\times -449.579)]-[(2\times -357.05)+(1\times 69.602)]=-318.618kJ](/tpl/images/0528/4508/7eefc.png)


