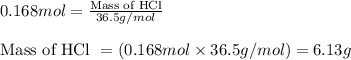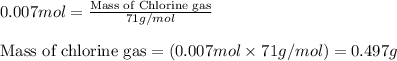Law of Conservation of Mass Assigned as Homework HQ4.01 In the following chemical reaction between H2 and Cla to produce HCl, what is the sum of the mass of HCl produced plus the mass of left over reactants when 3.36E-1 g of H2 completely reacts with 1.245E+1 g of Cl2? H2(g) + Cl2 (g) → 2HCl(g) T Respond with the correct number of significant figures in scientific notation

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1


Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1
You know the right answer?
Law of Conservation of Mass Assigned as Homework HQ4.01 In the following chemical reaction between H...
Questions

English, 22.06.2019 09:30




English, 22.06.2019 09:30





Business, 22.06.2019 09:30

History, 22.06.2019 09:30

History, 22.06.2019 09:30



Chemistry, 22.06.2019 09:30

History, 22.06.2019 09:30



Geography, 22.06.2019 09:30

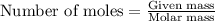 .....(1)
.....(1)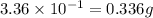
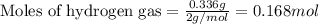
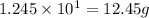
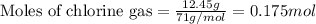
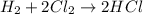
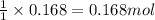 of chlorine gas
of chlorine gas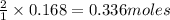 of HCl
of HCl