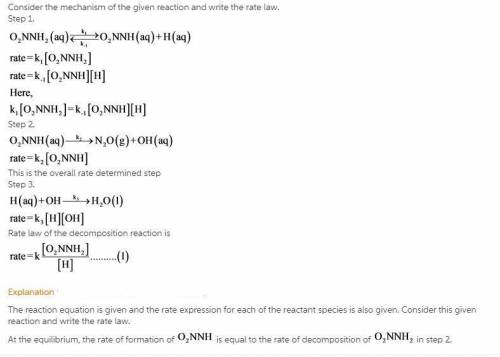
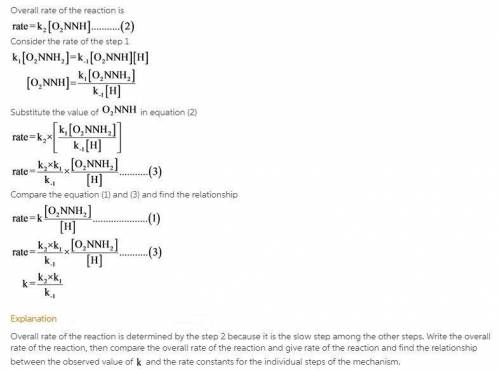
The decomposition of nitramide, O 2 NNH 2 , in water has the chemical equation and rate law O 2 NNH 2 ( aq ) ⟶ N 2 O ( g ) + H 2 O ( l ) rate = k [ O 2 NNH 2 ] [ H + ] A proposed mechanism for this reaction is O 2 NNH 2 ( aq ) k 1 ⇌ k − 1 O 2 NNH − ( aq ) + H + ( aq ) ( fast equilibrium ) O 2 NNH − ( aq ) k 2 −→ N 2 O ( g ) + OH − ( aq ) ( slow ) H + ( aq ) + OH − ( aq ) k 3 −→ H 2 O ( l ) ( fast ) What is the relationship between the observed value of k and the rate constants for the individual steps of the mechanism?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1


Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
The decomposition of nitramide, O 2 NNH 2 , in water has the chemical equation and rate law O 2 NNH...
Questions



History, 19.10.2021 01:00


Mathematics, 19.10.2021 01:00

History, 19.10.2021 01:00

Mathematics, 19.10.2021 01:00



Mathematics, 19.10.2021 01:00

History, 19.10.2021 01:00


Health, 19.10.2021 01:00




Law, 19.10.2021 01:00

Chemistry, 19.10.2021 01:00

Mathematics, 19.10.2021 01:00