
Chemistry, 27.02.2020 01:54 psychocatgirl1
Consider the reaction of ethyl acetate with sodium hydroxide: CH3COOC2H5(aq)+NaOH(aq)⇌CH3COONa(aq )+C2H5OH(aq) The reaction is first order in NaOH and second order overall. What is the rate law? View Available Hint(s) Consider the reaction of ethyl acetate with sodium hydroxide: The reaction is first order in and second order overall. What is the rate law? rate=k[CH3COOC2H5]2[NaOH]2 rate=k[CH3COOC2H5][NaOH]2 rate=k[NaOH] rate=k[CH3COOC2H5] rate=k[CH3COOC2H5][NaOH] rate=k[CH3COOC2H5]2[NaOH]

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Match the following items. 1. high-intensity bundle of energy being emitted from some decaying nuclei gamma ray 2. particle radiating from the nucleus of some atoms beta particle 3. negative particle identical to an electron but radiating from a decaying nucleus alpha particle
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3


Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
Consider the reaction of ethyl acetate with sodium hydroxide: CH3COOC2H5(aq)+NaOH(aq)⇌CH3COONa(aq )+...
Questions



History, 11.10.2019 14:10


Chemistry, 11.10.2019 14:10

Biology, 11.10.2019 14:10



Mathematics, 11.10.2019 14:10





Biology, 11.10.2019 14:10


Mathematics, 11.10.2019 14:10

Mathematics, 11.10.2019 14:10


History, 11.10.2019 14:10

Mathematics, 11.10.2019 14:10

![rate=k[CH_3COOC_2H_5]^1[NaOH]^1](/tpl/images/0526/0774/1e7fe.png)
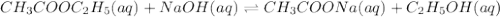
![rate=k[CH_3COOC_2H_5]^x[NaOH]^y](/tpl/images/0526/0774/4cba9.png)
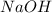
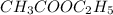 is 1 and rate law is :
is 1 and rate law is : 

