
A voltaic cell is set up with copper and hydrogen half-cells. Standard conditions are used in the copper half-cell, Cu2+ (aq, 1.00 M) | Cu (s). The hydrogen gas pressure is 1.00 bar. A value of 0.490 V is recorded for E Cell at 298 K. Determine the concentration of H+ and the pH of the solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 16:50
Which of the following is an indication that a substance has undergone a chemical change? a. no new product has been formed. b. the color of the substance has not changed. c. the original constitute has not changed. d. the molecular structure has changed.
Answers: 1
You know the right answer?
A voltaic cell is set up with copper and hydrogen half-cells. Standard conditions are used in the co...
Questions

SAT, 23.12.2021 01:00






Biology, 23.12.2021 01:00








Mathematics, 23.12.2021 01:00





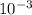 M and the pH = 2.6 of the solution
M and the pH = 2.6 of the solution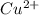 ) is the cathode and hydrogen (
) is the cathode and hydrogen (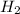 ) is the anode.
) is the anode.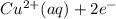 ⇒ Cu(s)
⇒ Cu(s)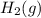 ⇒
⇒ 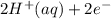
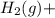
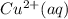 ⇒
⇒ 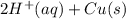
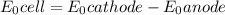
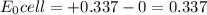
![\frac{[H^{+}]^{2} }{[Cu^{2+}]P_{H2} }](/tpl/images/0525/6315/22043.png)
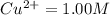 but
but 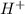 is unknown. we solve this using hernst equation.
is unknown. we solve this using hernst equation.![E = E^{0} -\frac{0.0257}{n}ln\frac{[H^{+}]^{2} }{[Cu^{2+}]P_{H2} }](/tpl/images/0525/6315/8e268.png)
![0.490 = 0.337 -\frac{0.0257}{2}ln\frac{[H^{+}]^{2} }{[1][1]}](/tpl/images/0525/6315/43505.png)
![ln{[H^{+}]^{2} } = -11.9](/tpl/images/0525/6315/8c81e.png)
![2ln{[H^{+}] } = -11.9](/tpl/images/0525/6315/31a40.png)
![ln{[H^{+}] } = -5.95](/tpl/images/0525/6315/e2ee5.png)
![[H^{+}] = 3* 10^{-3} M](/tpl/images/0525/6315/d9bb9.png)


