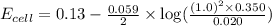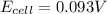Chemistry, 26.02.2020 20:54 adazeb2003
Calculate Ecell for the following electrochemical cell at 25 ºCPt (s) | H2 (g, 1.00 atm) | H+ (aq, 1.00 M) || Sn2+ (aq, 0.350 M) | Sn4+ (aq, 0.020 M) | Pt (s)The standard reduction potentials are as follows:Sn4+ (aq) + 2 e–à Sn2+ (aq) Eº = +0.13 V2 H+ (aq) + 2 e–à H2 (g) Eº = 0.00 V

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
Calculate Ecell for the following electrochemical cell at 25 ºCPt (s) | H2 (g, 1.00 atm) | H+ (aq, 1...
Questions

Computers and Technology, 21.04.2020 17:24



Engineering, 21.04.2020 17:24





Arts, 21.04.2020 17:24


Mathematics, 21.04.2020 17:24

Mathematics, 21.04.2020 17:24





Mathematics, 21.04.2020 17:24


Mathematics, 21.04.2020 17:24

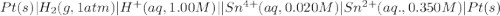
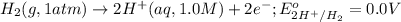
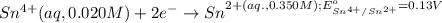
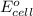 of the reaction, we use the equation:
of the reaction, we use the equation: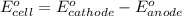
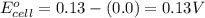
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[H^{+}]^2[Sn^{2+}]}{[Sn^{4+}]}](/tpl/images/0525/4106/69569.png)
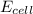 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![[H^{+}]=1.00M](/tpl/images/0525/4106/641ea.png)
![[Sn^{2+}]=0.350M](/tpl/images/0525/4106/7d5cb.png)
![[Sn^{4+}]=0.020M](/tpl/images/0525/4106/c69f3.png)