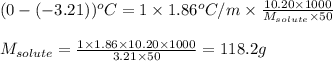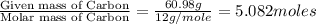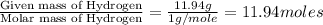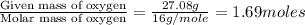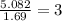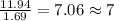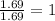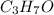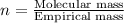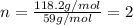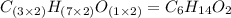Chemistry, 12.02.2020 04:42 Inrimid3619
A solution contains 10.20 g of unknown compound (non-electrolyte) dissolved in 50.0 mL of water. (Assume a density of 1.00 g/mL for water.) The freezing point of the solution is -3.21 ∘C. The mass percent composition of the compound is 60.98% C, 11.94% H, and the rest is ?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
A solution contains 10.20 g of unknown compound (non-electrolyte) dissolved in 50.0 mL of water. (As...
Questions















Computers and Technology, 11.02.2020 02:15



Computers and Technology, 11.02.2020 02:15

History, 11.02.2020 02:15

English, 11.02.2020 02:15

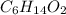
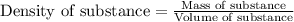
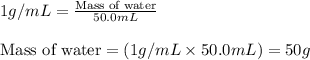
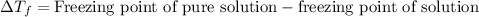
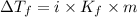
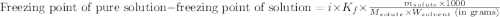
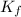 = molal boiling point elevation constant = 1.86°C/m
= molal boiling point elevation constant = 1.86°C/m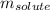 = Given mass of solute = 10.20 g
= Given mass of solute = 10.20 g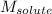 = Molar mass of solute = ?
= Molar mass of solute = ?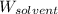 = Mass of solvent (water) = 50.0 g
= Mass of solvent (water) = 50.0 g