Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Nuclear fusion is the source of energy for stars. besides hydrogen, which other element is most likely also common in stars?
Answers: 1

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

You know the right answer?
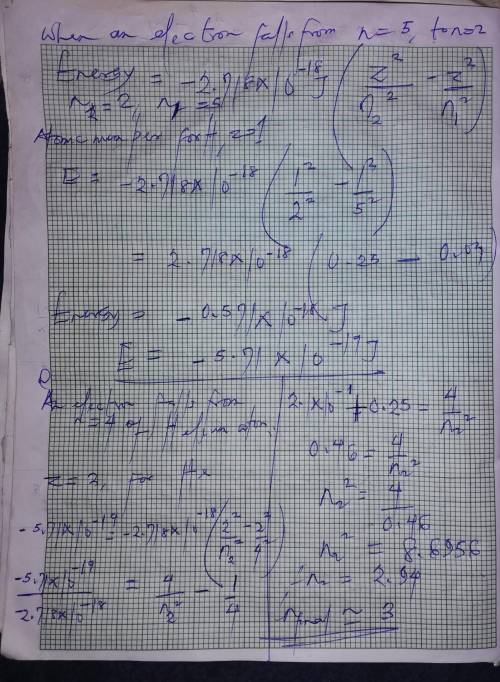
When the excited electron in a hydrogen atom falls from to , a photon of blue light is emitted. if a...
Questions


History, 11.07.2019 10:30





Computers and Technology, 11.07.2019 10:30




Mathematics, 11.07.2019 10:30

Mathematics, 11.07.2019 10:30



History, 11.07.2019 10:30

Mathematics, 11.07.2019 10:30




History, 11.07.2019 10:30