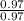Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

You know the right answer?
An unknown compound contains 75.69% carbon, 8.80% hydrogen, and 15.51% oxygen by mass. a mass spectr...
Questions


English, 24.12.2019 07:31

History, 24.12.2019 07:31

Computers and Technology, 24.12.2019 07:31

English, 24.12.2019 07:31


History, 24.12.2019 07:31













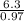 H -
H -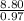 O -
O -