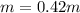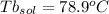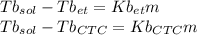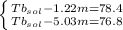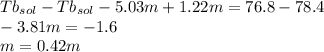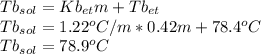Chemistry, 16.01.2020 17:31 naomicervero
Imagine two solutions with the same concentration and the same boiling point, but one has ethanol as the solvent and the other has carbon tetrachloride as the solvent. determine that molal concentration, m (or b ), and boiling point, tb. given: ethanolnormal boiling point: 78.4kb: 1.22ccl4normal boiling point: 76.8kb: 5.03

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1
You know the right answer?
Imagine two solutions with the same concentration and the same boiling point, but one has ethanol as...
Questions



History, 26.11.2019 00:31



History, 26.11.2019 00:31


Chemistry, 26.11.2019 00:31


Geography, 26.11.2019 00:31




Mathematics, 26.11.2019 00:31




Computers and Technology, 26.11.2019 00:31

Mathematics, 26.11.2019 00:31