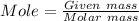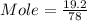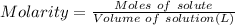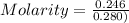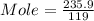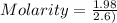What is the molarity (m) of the following solutions?
a. 19.2 g of al(oh)3 dissolved in w...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Questions







Mathematics, 17.04.2021 02:00

Mathematics, 17.04.2021 02:00

Mathematics, 17.04.2021 02:00


Mathematics, 17.04.2021 02:00

Mathematics, 17.04.2021 02:00


English, 17.04.2021 02:00




Mathematics, 17.04.2021 02:00

Mathematics, 17.04.2021 02:00

Physics, 17.04.2021 02:00

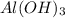 = 78 g/mole
= 78 g/mole