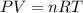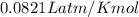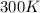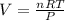Chemistry, 17.04.2021 02:00 Shavaila18
4. If 52.0 g of magnesium react with excess hydrochloric acid, how many liters of hydrogen gas can be made at 300 K and 0.970 atm?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
4. If 52.0 g of magnesium react with excess hydrochloric acid, how many liters of hydrogen gas can b...
Questions





Computers and Technology, 24.06.2020 17:01



Computers and Technology, 24.06.2020 17:01









Mathematics, 24.06.2020 17:01


Mathematics, 24.06.2020 17:01

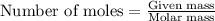
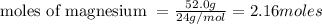
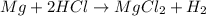
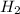
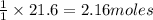 of
of