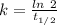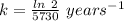Chemistry, 16.12.2019 18:31 bcifuentes
The previous part could be done without using the decay equation, because the ratio of original 14c to present 14c was an integer power of 1/2. most problems are not so simple. to solve more general carbon-dating problems, you must first find the value of the decay constant for 14c, so that you can easily use the decay equation. using the given half-life, 5730 years, find the value of the decay constant for 14c.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
The previous part could be done without using the decay equation, because the ratio of original 14c...
Questions


Mathematics, 20.07.2019 19:30



Chemistry, 20.07.2019 19:30




Mathematics, 20.07.2019 19:30

Arts, 20.07.2019 19:30

Mathematics, 20.07.2019 19:30


History, 20.07.2019 19:30

Biology, 20.07.2019 19:30






Mathematics, 20.07.2019 19:30

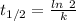
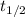 is the half life
is the half life