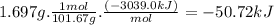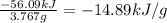Chemistry, 14.12.2019 03:31 mahmudabiazp3ekot
At constant volume, the heat of combustion of a particular compound, compound a, is − 3039.0 kj / mol. when 1.697 g of compound a (molar mass = 101.67 g / mol ) is burned in a bomb calorimeter, the temperature of the calorimeter (including its contents) rose by 3.661 ∘ c. what is the heat capacity (calorimeter constant) of the calorimeter? c = kj/°c suppose a 3.767 g sample of a second compound, compound b, is combusted in the same calorimeter, and the temperature rises from 23.23 ∘ c to 27.28 ∘ c. what is the heat of combustion per gram of compound b?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1


Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
You know the right answer?
At constant volume, the heat of combustion of a particular compound, compound a, is − 3039.0 kj / mo...
Questions



Mathematics, 28.05.2020 16:59






Mathematics, 28.05.2020 17:00

Mathematics, 28.05.2020 17:00


Mathematics, 28.05.2020 17:00



History, 28.05.2020 17:00

Mathematics, 28.05.2020 17:00




Mathematics, 28.05.2020 17:00