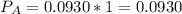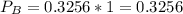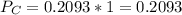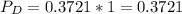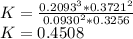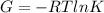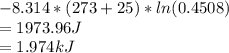Chemistry, 13.12.2019 01:31 Bryson2148
In the gas-phase reaction 2 a + b ⇌ 3 c + 2 d, it was found that, when 1.00 mol a, 2.00 mol b, and 1.00 mol d were mixed and allowed to come to equilibrium at 25 °c, the resulting mixture contained 0.90 mol c at a total pressure of 1.00 bar. calculate
(i) the mole fractions of each species at equilibrium,
(ii) k, and
(iii) δrimage.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

You know the right answer?
In the gas-phase reaction 2 a + b ⇌ 3 c + 2 d, it was found that, when 1.00 mol a, 2.00 mol b, and 1...
Questions

Mathematics, 13.10.2020 20:01

Social Studies, 13.10.2020 20:01

Mathematics, 13.10.2020 20:01

English, 13.10.2020 20:01


Mathematics, 13.10.2020 20:01

Mathematics, 13.10.2020 20:01






Physics, 13.10.2020 20:01


Computers and Technology, 13.10.2020 20:01

Mathematics, 13.10.2020 20:01




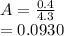
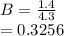
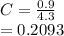
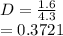
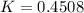
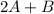 ⇄
⇄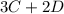
 be the number of moles dissociated per mole of
be the number of moles dissociated per mole of 
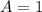
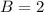
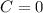
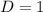
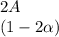 +
+ 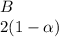 ⇄
⇄ 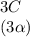 +
+ 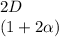
![C[tex] is 0.9Thus ,[tex]3\alpha=0.9\\\alpha=0.3[tex]The final number of moles of:[tex]A = 1-2\alpha=1-2*0.3=0.4mol[tex] [tex]B=2(1-\alpha)=2(1-0.3)=1.4mol[tex][tex]D=1+2\alpha=1+2*0.3=1.6mol[tex]Thus , total number of moles are : 0.4+1.4+0.9+1.6=4.3(i)The mole fractions are : [tex]A=\frac{0.4}{4.3} \\=0.0930](/tpl/images/0416/2107/4a2fd.png)
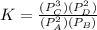
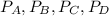 are the partial pressures of A,B,C,D respectively.
are the partial pressures of A,B,C,D respectively.