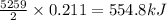Chemistry, 12.12.2019 06:31 alexdziob01
)b5h9(l) is a colorless liquid that will explode when exposed to oxygen. how much heat is released when 0.211 mol of b5h9 reacts with excess oxygen where the products are b2o3(s) and h2o(l). the standard enthalpy of formation of b5h9(l) is 73.2 kj/mol, the standard enthalpy of formation of b2o3(s) is -1272 kj/mol and that of h2o(l) is -285.4 kj/mol. express your answer in kj.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which mathematical relationship should you us to convert moles of a substance into grams
Answers: 1

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
)b5h9(l) is a colorless liquid that will explode when exposed to oxygen. how much heat is released w...
Questions

Mathematics, 31.03.2020 12:49

History, 31.03.2020 12:50



Physics, 31.03.2020 12:51

Mathematics, 31.03.2020 12:52





Mathematics, 31.03.2020 12:57



History, 31.03.2020 12:59

Mathematics, 31.03.2020 13:00


English, 31.03.2020 13:04

Mathematics, 31.03.2020 13:04


Social Studies, 31.03.2020 13:04

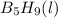 reacts is 554.8 kJ
reacts is 554.8 kJ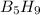 with oxygen gas follows:
with oxygen gas follows: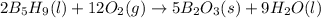
![\Delta H_{rxn}=[(5\times \Delta H_f_{(B_2O_3(s))})+(9\times \Delta H_f_{(H_2O(l))})]-[(2\times \Delta H_f_{(B_5H_9(l))})+(12\times \Delta H_f_{(O_2(g))})]](/tpl/images/0414/9485/05a9d.png)
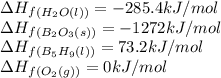
![\Delta H_{rxn}=[(2\times (-1272))+(9\times (-285.4))]-[(2\times (73.2))+(12\times (0))]\\\\\Delta H_{rxn}=-5259kJ](/tpl/images/0414/9485/eaac9.png)