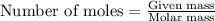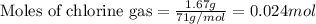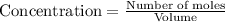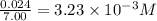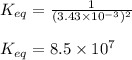Titanium and chlorine react to form titanium(iv) chloride, like this: ti(s) + 2 cl 2(g)-ticl 4( at a certain temperature, a chemist finds that a 7.0 l reaction vessel containing a mixture of titanium, chlorine, and titanium(iv) chloride at equilibrium has the following composition compound amount 1.67 g cl 2.93 g tici 2.02 g ti calculate the value of the equilibrium constant k for this reaction. round your answer to 2 significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

You know the right answer?
Titanium and chlorine react to form titanium(iv) chloride, like this: ti(s) + 2 cl 2(g)-ticl 4( at...
Questions

Mathematics, 07.01.2020 06:31

Mathematics, 07.01.2020 06:31




History, 07.01.2020 06:31

English, 07.01.2020 06:31



Mathematics, 07.01.2020 06:31


Biology, 07.01.2020 06:31




Mathematics, 07.01.2020 06:31


Social Studies, 07.01.2020 06:31

Mathematics, 07.01.2020 06:31

History, 07.01.2020 06:31


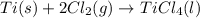
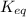 for the above reaction follows:
for the above reaction follows:![K_{eq}=\frac{1}{[Cl_2]^2}](/tpl/images/0413/8706/8ac7b.png)