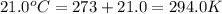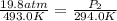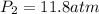Chemistry, 10.12.2019 21:31 payshencec21
A20.6-l sample of "pure" air is collected in greenland at a temperature of 220.0°c and a pressure of 1.01 atm and is forced into a 1.05-l bottle for shipment to europe for analysis.
(a) compute the pressure inside the bottle just after it is filled.
(b) compute the pressure inside the bottle as it is opened in the 21.0°c comfort of the european laboratory.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 08:00
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1
You know the right answer?
A20.6-l sample of "pure" air is collected in greenland at a temperature of 220.0°c and a pressure of...
Questions


History, 12.02.2022 07:20

History, 12.02.2022 07:20


Advanced Placement (AP), 12.02.2022 07:20




History, 12.02.2022 07:20




Mathematics, 12.02.2022 07:20

Biology, 12.02.2022 07:20

Spanish, 12.02.2022 07:20

Mathematics, 12.02.2022 07:20

Mathematics, 12.02.2022 07:20

Computers and Technology, 12.02.2022 07:20


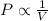
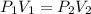
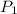 = initial pressure of gas = 1.01 atm
= initial pressure of gas = 1.01 atm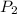 = final pressure of gas = ?
= final pressure of gas = ?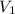 = initial volume of gas = 20.6 L
= initial volume of gas = 20.6 L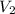 = final volume of gas = 1.05 L
= final volume of gas = 1.05 L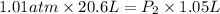
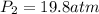
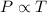
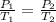
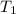 = initial temperature of gas =
= initial temperature of gas = 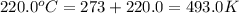
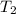 = final temperature of gas =
= final temperature of gas =