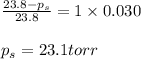Chemistry, 10.12.2019 01:31 romaguera06
Determine the vapor pressure of a solution at 25°c that contains 76.6 g of glucose (c6h12o6) in 250.0 ml of water. the vapor pressure of pure water at 25°c is 23.8 torr.70.8 torr7.29 torr72.9 torr22.9 torr23.1 torr

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 23.06.2019 01:00
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 10:00
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1

You know the right answer?
Determine the vapor pressure of a solution at 25°c that contains 76.6 g of glucose (c6h12o6) in 250....
Questions


Physics, 16.12.2021 04:50

Mathematics, 16.12.2021 04:50



Mathematics, 16.12.2021 04:50


Computers and Technology, 16.12.2021 04:50


English, 16.12.2021 04:50

Mathematics, 16.12.2021 04:50

Biology, 16.12.2021 04:50

Biology, 16.12.2021 04:50


English, 16.12.2021 04:50



Mathematics, 16.12.2021 04:50

Computers and Technology, 16.12.2021 04:50

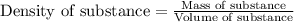
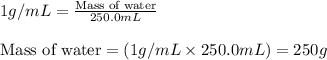
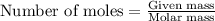 .....(1)
.....(1)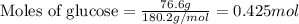
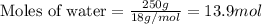
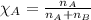
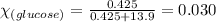
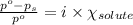
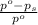 = relative lowering in vapor pressure
= relative lowering in vapor pressure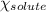 = mole fraction of solute = 0.030
= mole fraction of solute = 0.030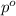 = vapor pressure of pure water = 23.8 torr
= vapor pressure of pure water = 23.8 torr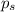 = vapor pressure of solution = ?
= vapor pressure of solution = ?