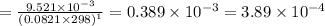Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
Consider the following equilibrium.2 nobr(g)< => 2 no(g) + br2(g)if nitrosyl bromide, nobr, i...
Questions


History, 21.10.2019 21:00

Mathematics, 21.10.2019 21:00



Mathematics, 21.10.2019 21:00



Computers and Technology, 21.10.2019 21:00


Mathematics, 21.10.2019 21:00


Mathematics, 21.10.2019 21:00

Social Studies, 21.10.2019 21:00


Chemistry, 21.10.2019 21:00


English, 21.10.2019 21:00


Mathematics, 21.10.2019 21:00

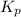 of the reaction is
of the reaction is 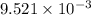 .
.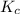 of the reaction is
of the reaction is 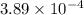 .
.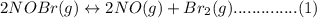
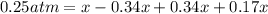
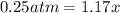
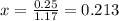
![[]P_{NOBr}]](/tpl/images/0401/8095/a6c6d.png) is 0.14 atm.
is 0.14 atm.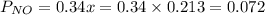
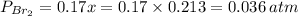
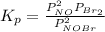
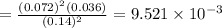
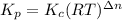
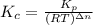
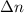 = number of moles of reactants - Number of moles of products
= number of moles of reactants - Number of moles of products