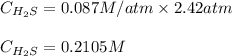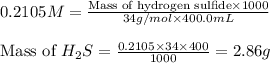Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 12:40
How can you increase the ability of a gas to dissolve in a liquids?
Answers: 1

Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
At 25.0 ⁰c the henry's law constant for hydrogen sulfide(h2s) gas in water is 0.087 m/atm. caculate...
Questions


Mathematics, 03.08.2019 15:50


Physics, 03.08.2019 15:50

Physics, 03.08.2019 15:50

Physics, 03.08.2019 15:50

Physics, 03.08.2019 15:50




Business, 03.08.2019 15:50

Biology, 03.08.2019 15:50



Social Studies, 03.08.2019 15:50

Biology, 03.08.2019 15:50

Social Studies, 03.08.2019 15:50

Chemistry, 03.08.2019 15:50


History, 03.08.2019 15:50

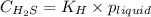
 = Henry's constant =
= Henry's constant = 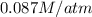
 = partial pressure of hydrogen sulfide gas = 2.42 atm
= partial pressure of hydrogen sulfide gas = 2.42 atm