
An element crystallizes in a face centered cubic lattice and has a density of 1.45 g cm-3 . the edge of its unit cell is 4.52 x 10-8cm.
a) how many atoms are in each unit cell?
b) what is the volume of a unit cell?
c) what is the mass of a unit cell?
d) calculate the approximate atomic mass of the element.

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

You know the right answer?
An element crystallizes in a face centered cubic lattice and has a density of 1.45 g cm-3 . the edge...
Questions


Geography, 27.02.2021 14:00



Biology, 27.02.2021 14:00

Law, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00




Spanish, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00


World Languages, 27.02.2021 14:00

Biology, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00

Mathematics, 27.02.2021 14:00



Mathematics, 27.02.2021 14:00

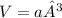 , a being the edge length.
, a being the edge length.

