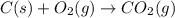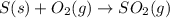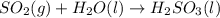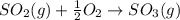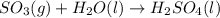In ontario, some electricity comes from coal-burning generators. coal is a natural form of carbon that has a large amount of sulphur mixed in with it. answer the following questions based on the burning of coal to produce energy.
a) write the word equations and balanced chemical equations for the burning of carbon and the burning of sulphur. (4 marks)
b) which of these products is harmful to the environment? how is it harmful? (2 marks)
c) write the word equation and balanced chemical equation for the reaction that produces this harmful environmental effect. (2 marks)
d)explain why it is important to make sure your furnace is tuned up and in proper working order before the winter

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
In ontario, some electricity comes from coal-burning generators. coal is a natural form of carbon th...
Questions

Business, 27.06.2020 18:01


Mathematics, 27.06.2020 18:01



Mathematics, 27.06.2020 18:01


Mathematics, 27.06.2020 18:01

Mathematics, 27.06.2020 18:01





History, 27.06.2020 18:01

Mathematics, 27.06.2020 18:01




Computers and Technology, 27.06.2020 18:01