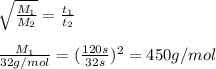Chemistry, 30.11.2019 01:31 hannahbeccahxo9681
Agas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. it required 120 s for 1.0 l of the gas to effuse. under identical experimental conditions it required 32 s for 1.0 l of o2 gas to effuse. you may want to reference (pages 416 - 419) section 10.8 while completing this problem. part a calculate the molar mass of the unknown gas. (remember that the faster the rate of effusion, the shorter the time required for effusion of 1.0 l; that is, rate and time are inversely proportional.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2
You know the right answer?
Agas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure...
Questions

Chemistry, 18.09.2019 10:50


Mathematics, 18.09.2019 10:50




Health, 18.09.2019 10:50


History, 18.09.2019 10:50



History, 18.09.2019 10:50

History, 18.09.2019 10:50


Chemistry, 18.09.2019 10:50



History, 18.09.2019 10:50


Mathematics, 18.09.2019 10:50