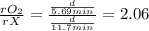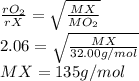Asample of o2 gas is observed to effuse through a porous barrier in 5.69 minutes. under
the sa...

Chemistry, 30.11.2019 01:31 jeanette7482
Asample of o2 gas is observed to effuse through a porous barrier in 5.69 minutes. under
the same conditions, the same number of moles of an unknown gas requires 11.7 minutes
to effuse through the same barrier. what is the molar mass of the unknown gas?
a) 11.6 g/mol
b) 135 g/mol
c) 7.56 g/mol
d) 66.5 g/mol
e) more information is needed

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
Questions

Mathematics, 12.07.2019 23:10


Mathematics, 12.07.2019 23:10


Mathematics, 12.07.2019 23:10









Computers and Technology, 12.07.2019 23:10





Computers and Technology, 12.07.2019 23:10