If you are given an ideal gas with pressure (p) = 259,392.00 pa and temperature (t) = 2.00 oc
...


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2

Chemistry, 22.06.2019 23:00
What prefix multiplier is appropriate for reporting a measurement of 5.57 ×10−5 m?
Answers: 1

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3

Chemistry, 23.06.2019 13:40
Which of the following volumes is the smallest? a) one microliter b)one deciliter d)one liter c)one milliliter
Answers: 2
You know the right answer?
Questions



Chemistry, 14.06.2021 21:50

English, 14.06.2021 21:50

Mathematics, 14.06.2021 21:50




Social Studies, 14.06.2021 21:50


Mathematics, 14.06.2021 21:50

Mathematics, 14.06.2021 21:50

Mathematics, 14.06.2021 21:50



Mathematics, 14.06.2021 21:50




Mathematics, 14.06.2021 21:50

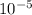 atm
atm

