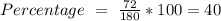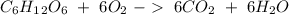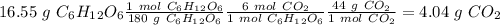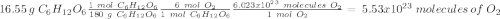Glucose (c6h12o6) is a key nutrient for generating chemical potential energy in biological systems. we were provided 16.55 g of glucose. calculate:
a) the mass percent of carbon in glucose.
b) the mass of co2 produced by the combustion of 16.55 g glucose with sufficient oxygen gas.
c) how many oxygen molecules needed for the completely combustion of 16.55 g glucose?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1
You know the right answer?
Glucose (c6h12o6) is a key nutrient for generating chemical potential energy in biological systems....
Questions


Mathematics, 22.04.2020 21:54




Biology, 22.04.2020 21:54

Physics, 22.04.2020 21:54

Mathematics, 22.04.2020 21:54


Mathematics, 22.04.2020 21:54


Mathematics, 22.04.2020 21:54



Mathematics, 22.04.2020 21:55


Geography, 22.04.2020 21:55

Chemistry, 22.04.2020 21:55


Mathematics, 22.04.2020 21:55

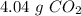
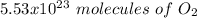
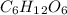 , so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.
, so the first step is to find the atomic mass of each atom and multiply by the amount of atoms in the molecule.