
Chemistry, 23.11.2019 05:31 lazavionadams81
Find an expression for the change in entropy when two blocks of the same substance of equal mass, one at the temperature th and the other at tc, are brought into contact and allowed to reach equilibrium. evaluate the change for the two blocks of copper, each of mass 500 grams with cpcm= 24.4 j kt-1 mol-1, taking th = 500 k and tc = 250 k.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 23.06.2019 01:30
Astudent states that 9.0 g of baking soda will form an unsaturated solution in 100 g of water. what do you need to know to decide whether this statement is correct? a. the temperature of the water and the molar mass of baking soda b. the percent by volume of the solution and the solubility of baking soda c. the temperature of the water and the solubility of baking soda at that temperature
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
Find an expression for the change in entropy when two blocks of the same substance of equal mass, on...
Questions


Computers and Technology, 15.11.2019 22:31








Biology, 15.11.2019 22:31

Mathematics, 15.11.2019 22:31

Mathematics, 15.11.2019 22:31




Chemistry, 15.11.2019 22:31




Biology, 15.11.2019 22:31

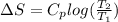
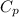 = 24.4 J/mol K
= 24.4 J/mol K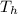 = 500 K,
= 500 K, 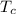 = 250 K
= 250 K 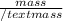
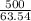
![7.86 \times 24.4 \times [T_{f} - 250]](/tpl/images/0387/6495/f5240.png) =
= ![7.86 \times 24.4 \times [500 -T_{f}]](/tpl/images/0387/6495/72b18.png)
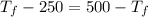
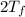 = 750
= 750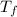 =
= 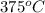
![24.4 log [\frac{375}{500}]](/tpl/images/0387/6495/8e24d.png)
![24.4 log [\frac{375}{250}]](/tpl/images/0387/6495/56e73.png)


