

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 21.06.2019 19:00
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1


Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
Which of the following transitions represent the emission of a photon with the largest energy?
Questions

Mathematics, 09.06.2021 20:30



Mathematics, 09.06.2021 20:30

English, 09.06.2021 20:30






Mathematics, 09.06.2021 20:30


English, 09.06.2021 20:30

Mathematics, 09.06.2021 20:30




Chemistry, 09.06.2021 20:30


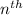 orbit is directly proportional to
orbit is directly proportional to 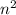 .Hence a transistion from one orbit to another orbit emits an energy proportional to the difference of their squares of the orbits. that is if an electron travels from orbit n1 to orbit n2 then it emits an energy corresponding to
.Hence a transistion from one orbit to another orbit emits an energy proportional to the difference of their squares of the orbits. that is if an electron travels from orbit n1 to orbit n2 then it emits an energy corresponding to  .So in the above question the highest energy emission occurs when an electron moves from n=6 to n=3.(Highest difference of energy levels).
.So in the above question the highest energy emission occurs when an electron moves from n=6 to n=3.(Highest difference of energy levels).

