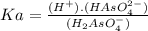Chemistry, 20.11.2019 05:31 carlos200411114
Write a balanced equation and ka expression for the brønsted-lowry acid h2aso4− in water. (in the balanced equation, be sure to indicate the state of each species and any charge associated with it.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

You know the right answer?
Write a balanced equation and ka expression for the brønsted-lowry acid h2aso4− in water. (in the ba...
Questions



Mathematics, 12.03.2020 05:08
















Biology, 12.03.2020 05:08

Biology, 12.03.2020 05:08