
Chemistry, 20.11.2019 00:31 brandon436
What is the ph of a buffer in which the concentration of benzoic acid, c6h5cooh, is 0.040 m and the concentration of sodium benzoate, nac6h5coo, is 0.015 m ? ka of c6h5cooh is 6.30 x 10-5

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

You know the right answer?
What is the ph of a buffer in which the concentration of benzoic acid, c6h5cooh, is 0.040 m and the...
Questions


Computers and Technology, 11.09.2019 05:10














Computers and Technology, 11.09.2019 05:10




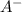 +
+ 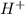
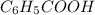 <=>
<=> 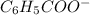 +
+ 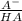
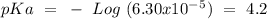
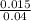 )
)

