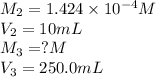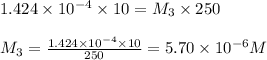Chemistry, 18.11.2019 18:31 sandeebassett3
In order to prepare very dilute solutions, a lab technician chooses to perform a series of dilutions instead of measuring a very small mass. a solution was prepared by dissolving 0.360 g of kno3 in enough water to make 500. ml of solution. a 10.0 ml sample of this solution was transferred to a 500.0-ml volumetric flask and diluted to the mark with water. then 10.0 ml of the diluted solution was transferred to a 250.0-ml flask and diluted to the mark with water. what is the final concentration of the kno3 solution? 7.91 × 10-9 m1.42 × 10-4 m5.70 × 10-6 m2.85 × 10-6 m7.12 × 10-3 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
In order to prepare very dilute solutions, a lab technician chooses to perform a series of dilutions...
Questions

Computers and Technology, 14.10.2019 20:50




History, 14.10.2019 20:50


History, 14.10.2019 21:00


Mathematics, 14.10.2019 21:00




Biology, 14.10.2019 21:00


Biology, 14.10.2019 21:00





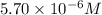
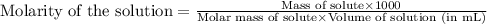
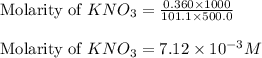
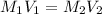 .......(1)
.......(1)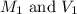 are the molarity and volume of the concentrated
are the molarity and volume of the concentrated 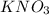 solution
solution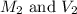 are the molarity and volume of diluted
are the molarity and volume of diluted 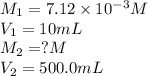
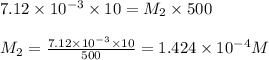
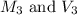 are the molarity and volume of diluted
are the molarity and volume of diluted