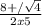Chemistry, 14.10.2019 21:00 missdee6929
H2(g) + i2(g) â 2 hi(g) kc = 64 at 400âºc 3.00 moles of h2 and 3.00 moles of i2 are placed in a 4.00 l container and the system is allowed to reach equilibrium. calculate the concentration of hi at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
H2(g) + i2(g) â 2 hi(g) kc = 64 at 400âºc 3.00 moles of h2 and 3.00 moles of i2 are placed in a 4.00...
Questions


Mathematics, 20.05.2021 09:50

Social Studies, 20.05.2021 09:50

Computers and Technology, 20.05.2021 09:50

Mathematics, 20.05.2021 09:50



History, 20.05.2021 09:50

Social Studies, 20.05.2021 09:50




Mathematics, 20.05.2021 09:50

English, 20.05.2021 09:50






Computers and Technology, 20.05.2021 09:50

![Kc = \frac{{D}^dx[C]^c}{[A]^ax[B]^b}](/tpl/images/0319/5603/47fdd.png)
![Kc = \frac{[HI]^2}{[H2]x[I2]}](/tpl/images/0319/5603/fe065.png)