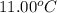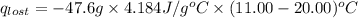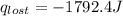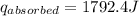A6.60 g sample of solid kcl was dissolved in 47.6 g of water. the initial temperature of the water was 20.00°c. after the compound dissolved, the temperature of the water was 11.00°c. assume the heat was completely absorbed from the water and no heat was absorbed by the reaction container or the surroundings. calculate the heat absorbed by the process. the specific heat of water is 4.184 j/g·°c.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1


Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
A6.60 g sample of solid kcl was dissolved in 47.6 g of water. the initial temperature of the water w...
Questions



Social Studies, 02.11.2020 08:50

Mathematics, 02.11.2020 08:50


English, 02.11.2020 08:50





Biology, 02.11.2020 08:50


History, 02.11.2020 08:50

Computers and Technology, 02.11.2020 08:50

Health, 02.11.2020 08:50

History, 02.11.2020 08:50


Business, 02.11.2020 08:50

Business, 02.11.2020 08:50

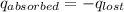
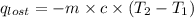
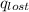 = heat lost by the water = ?
= heat lost by the water = ?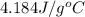
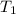 = initial temperature of water =
= initial temperature of water = 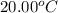
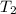 = final temperature of water =
= final temperature of water =