Chemistry, 08.11.2019 22:31 mercedesamatap21hx0
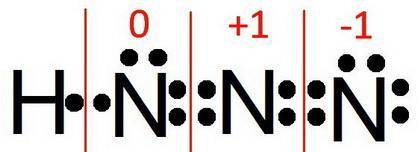
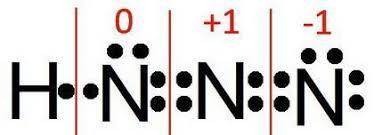
For a molecule of hydrazoic acid (hn3, also known as hydrogen azide), the atoms are arranged as hnnn. one resonance form of hn3 has a double bond between each of the nitrogen atoms: h−n=n=n what is the formal charge of each of the atoms in this resonance structure? notice that the formal charges are being asked in the same order as the atoms are listed in the formula. (hnanbnc)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
For a molecule of hydrazoic acid (hn3, also known as hydrogen azide), the atoms are arranged as hnnn...
Questions

History, 25.06.2019 20:00

History, 25.06.2019 20:00


Mathematics, 25.06.2019 20:00



Mathematics, 25.06.2019 20:00

Chemistry, 25.06.2019 20:00


Mathematics, 25.06.2019 20:00


Computers and Technology, 25.06.2019 20:00


Mathematics, 25.06.2019 20:00

Mathematics, 25.06.2019 20:00



English, 25.06.2019 20:00

Biology, 25.06.2019 20:00

Mathematics, 25.06.2019 20:00