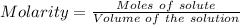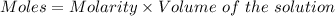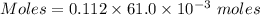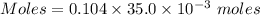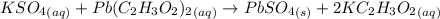A61.0ml sample of a 0.112m potassium sulfate solution is mixed with 35.0ml of a 0.104m lead(ii) acetate solution and the following precipitation reaction occurs:
k2so4(aq)+pb(c2h3o2)2(aq)? 2kc2h3o2(aq)+pbso4(s)
the solid pbso4 is collected, dried, and found to have a mass of 0.997g .
determine the limiting reactant, the theoretical yield, and the percent yield.
part a.
identify the limiting reactant.
kc2h3o2
pb(c2h3o2)2
k2so4
pbso4
part b.
determine the theoretical yield.
mass of pbso4 =
part c.
determine the percent yield=

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 02:00
In which of these cases are the two wave points considered to be in phase with each other?
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2
You know the right answer?
A61.0ml sample of a 0.112m potassium sulfate solution is mixed with 35.0ml of a 0.104m lead(ii) acet...
Questions

History, 29.06.2020 15:01


English, 29.06.2020 15:01

Mathematics, 29.06.2020 15:01

English, 29.06.2020 15:01

Mathematics, 29.06.2020 15:01

Social Studies, 29.06.2020 15:01


Social Studies, 29.06.2020 15:01



Medicine, 29.06.2020 15:01


Arts, 29.06.2020 15:01


English, 29.06.2020 15:01

Mathematics, 29.06.2020 15:01


English, 29.06.2020 15:01

Mathematics, 29.06.2020 15:01