
Chemistry, 04.11.2019 20:31 jakhunter354
Achemical reaction takes place inside a flask submerged in a water bath. the water bath contains 8.10kg of water at 33.9 degrees celsius . during the reaction 69.0kj of heat flows out of the bath and into the flask.
calculate the new temperature of the water bath. you can assume the specific heat capacity of water under these conditions is 4.18j*g*k. round your answer to 3 significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1
You know the right answer?
Achemical reaction takes place inside a flask submerged in a water bath. the water bath contains 8.1...
Questions



Computers and Technology, 17.02.2022 21:10


Biology, 17.02.2022 21:10



Mathematics, 17.02.2022 21:10


Mathematics, 17.02.2022 21:10

Social Studies, 17.02.2022 21:10



Chemistry, 17.02.2022 21:10



Social Studies, 17.02.2022 21:20


Health, 17.02.2022 21:20

Computers and Technology, 17.02.2022 21:20

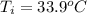 = (33.9 + 273) K = 306.9 K
= (33.9 + 273) K = 306.9 K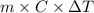
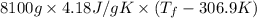
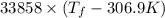
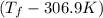 = 2.037 K
= 2.037 K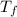 = (2.037 + 306.9) K
= (2.037 + 306.9) K

