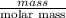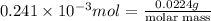Chemistry, 28.10.2019 21:31 alarconanais07
The following procedure provides a crude method of determining the molar mass of a volatile liquid. a liquid of mass 0.0224 g is introduced into a syringe and the end is capped (sealed). the syringe is transferred to a temperature bath maintained at 49.9 oc, and the liquid vaporizes. as the liquid vaporizes the plunger is pushed out. at equilibrium, the plunger reads 6.56 ml of gas. atmospheric pressure is 740. mmhg. what is the approximate molar mass of the compound (in g/mol)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 05:00
How many atomic mass units are equal to 1.672×10−24 g of protons?
Answers: 3
You know the right answer?
The following procedure provides a crude method of determining the molar mass of a volatile liquid....
Questions



Chemistry, 31.07.2019 11:30

Chemistry, 31.07.2019 11:30


Chemistry, 31.07.2019 11:30






Business, 31.07.2019 11:30




Social Studies, 31.07.2019 11:30



Biology, 31.07.2019 11:30

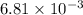 L
L
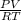
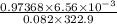
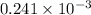 moles
moles