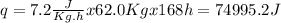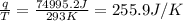Chemistry, 25.10.2019 04:43 elijahjacksonrp6z2o7
Q4: the average heat evolved by the oxidation of food in an average adult per hour per kilogram of body weight is 7.20 ୩ ୩ ୦୰. assume the weight of an average adult is 62.0 kkg. suppose the total heat evolved by this oxidation is tranderred into the surroundings over a period of 1 week. calculate the entropy change of the surroundings associated with this heat transfer. assume the surroundings are at 293k.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1


Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
Q4: the average heat evolved by the oxidation of food in an average adult per hour per kilogram of...
Questions

English, 10.02.2020 08:06

English, 10.02.2020 08:06



Mathematics, 10.02.2020 08:06

Biology, 10.02.2020 08:06

Mathematics, 10.02.2020 08:06

Biology, 10.02.2020 08:07


Biology, 10.02.2020 08:07



Chemistry, 10.02.2020 08:07

Advanced Placement (AP), 10.02.2020 08:07

Mathematics, 10.02.2020 08:08

Mathematics, 10.02.2020 08:08


Mathematics, 10.02.2020 08:08

History, 10.02.2020 08:08

Mathematics, 10.02.2020 08:09