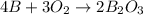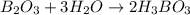Chemistry, 23.10.2019 18:30 jasmin2344
When elemental boron, b, is burned in oxygen gas, the product is diboron trioxide. if the diboron trioxide is then reacted with a measured quantity of water, it reacts with the water to form what is commonly known as boric acid, b(oh)3. write a balanced chemical equation for each of these processes. (use the lowest possible coefficients. omit states-of-matter in your answer.)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2
You know the right answer?
When elemental boron, b, is burned in oxygen gas, the product is diboron trioxide. if the diboron tr...
Questions



Mathematics, 22.09.2019 19:30

Mathematics, 22.09.2019 19:30

English, 22.09.2019 19:30



Mathematics, 22.09.2019 19:30

Mathematics, 22.09.2019 19:30

Biology, 22.09.2019 19:30



Biology, 22.09.2019 19:30

History, 22.09.2019 19:30

Social Studies, 22.09.2019 19:30



Mathematics, 22.09.2019 19:30

Biology, 22.09.2019 19:30

History, 22.09.2019 19:30