
Chemistry, 19.10.2019 00:30 laequity7325
Sodium thiosulfate, na2s2o3, is used as a fixer in photographic film developing. the amount of na2s2o3 in a solution can be determined by a titration with iodine, i2, according to the equation: 2na2s2o3(aq) + i2(aq) --> na2s4o6 +2nai(aq). calculate the concentration of the na2s2o3 solution if 23.50 ml of a 0.1710 m i2 solution react exactly with a 100.0 ml sample of the na2s2o3 solution. use 4 sig. fig. aside, the end of the titration is determined by the color. nai is a pale yellow and i2 is a deep purple. just when the purple color persists, all the iodine that can react has reacted.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:00
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 23.06.2019 00:30
Gasoline has a density of 0.740 g/ml. if you have 328 grams of gasoline, what is the volume in milliliters?
Answers: 1
You know the right answer?
Sodium thiosulfate, na2s2o3, is used as a fixer in photographic film developing. the amount of na2s2...
Questions

English, 28.12.2019 12:31

History, 28.12.2019 12:31




Business, 28.12.2019 13:31


Mathematics, 28.12.2019 13:31









Physics, 28.12.2019 13:31



Physics, 28.12.2019 13:31

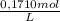 = 4,0185x10⁻³ moles of I₂
= 4,0185x10⁻³ moles of I₂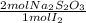 = 8,037x10⁻³ moles of Na₂S₂O₃
= 8,037x10⁻³ moles of Na₂S₂O₃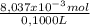 = 0,08037 M
= 0,08037 M

